What Is Quality Control Department In Pharmaceutical Industry Quality control is a critical component of the pharmaceutical industry focused on ensuring that pharmaceutical products meet established quality standards are safe for consumption and provide the intended therapeutic effect Quality control measures encompass a range of testing analysis and inspection procedures that are performed
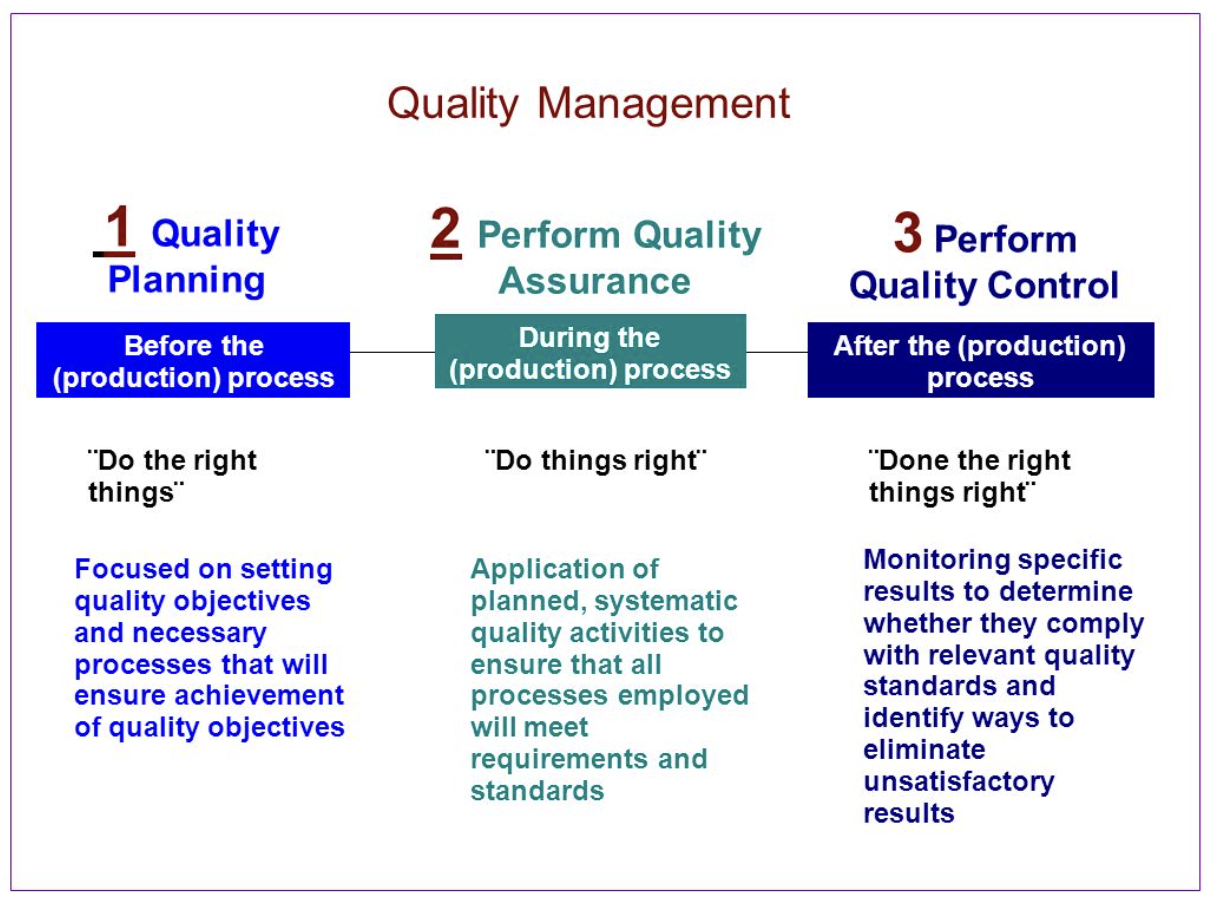
Physico Chemical Quality Control QC is one of the central departments in the pharmaceutical industry It ensures that the quality of incoming materials and outgoing products are of sufficient quality for their use or consumption In order to cover all the responsibilities of the Physico Chemical QC some companies separate this department Duration QA is a long term process that covers the entire development cycle while QC is more short term and happens at the end of the manufacturing process during the testing phase Steps QA may involve producing detailed documentation processing audit checklists and supplier management QC covers product sampling testing and inspections
What Is Quality Control Department In Pharmaceutical Industry
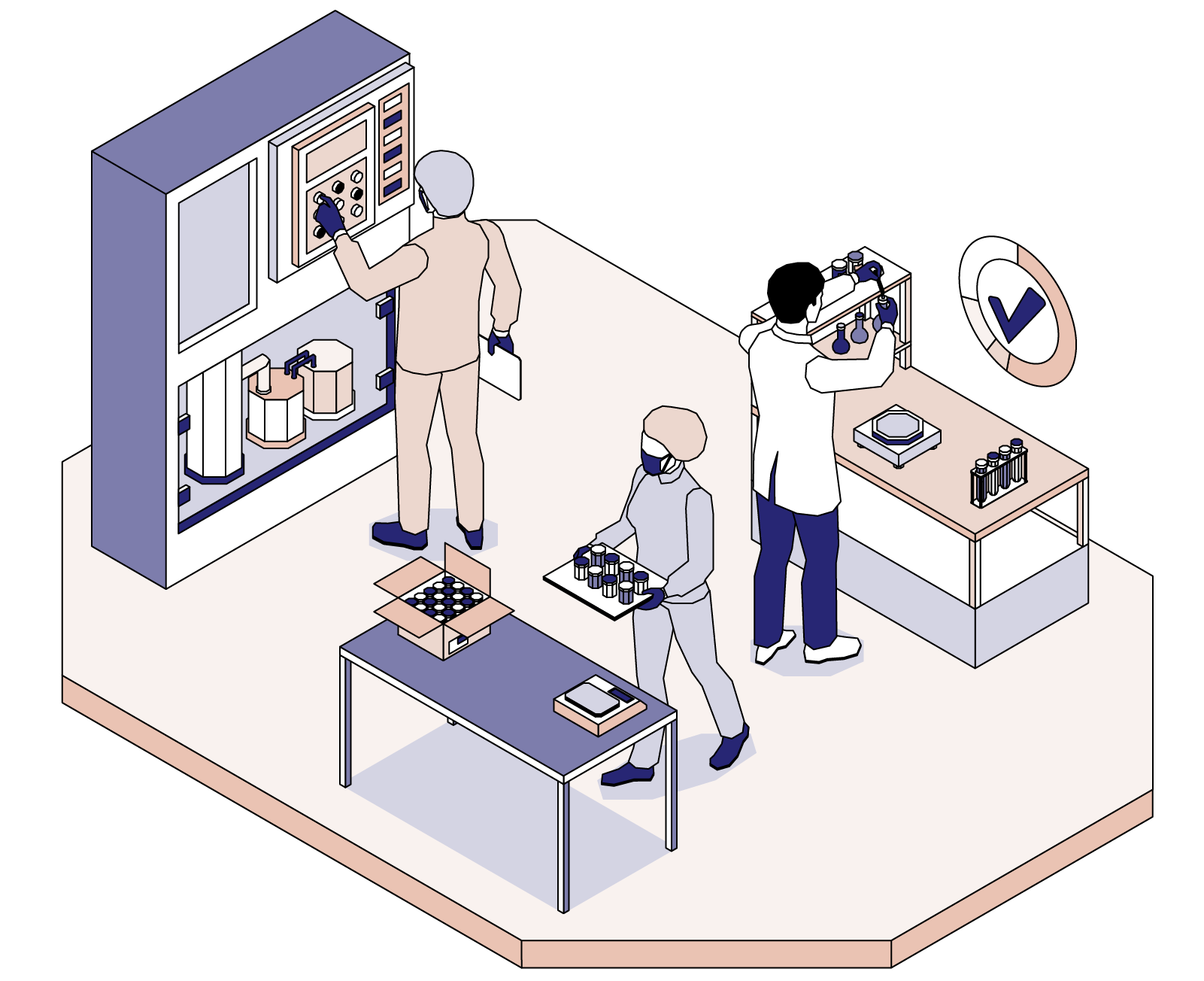
What Is Quality Control Department In Pharmaceutical Industry
https://amaris.com/wp-content/uploads/2022/07/The-importance-of-Quality-in-the-Pharmaceutical-Industry.png
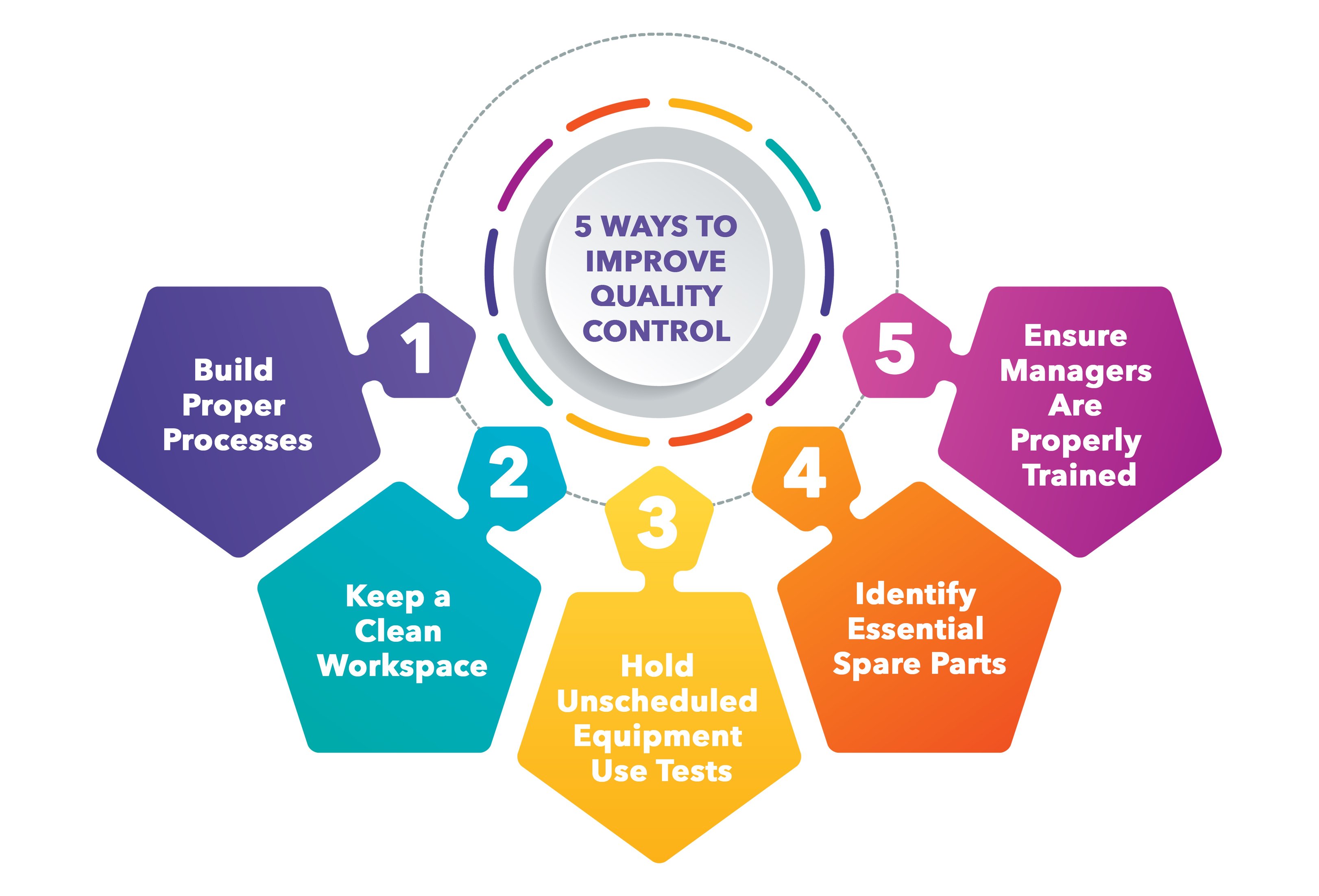
5 Ways To Improve Quality Control In Manufacturing
https://www.dozuki.com/hs-fs/hubfs/Imported_Blog_Media/Improve Quality Control in Manufacturing - infographic.jpeg?width=3243&name=Improve Quality Control in Manufacturing - infographic.jpeg

The Benefits Of Automation In Pharmaceutical Manufacturing
https://www.theengineer.co.uk/media/jl4bjtaw/eua540-1.jpg
The pharmaceutical quality control laboratory serves one of the most important functions in pharmaceutical production and control A significant portion of the CGMP regulations 21 CFR 211 Quality Production Laboratory Materials Facilities and Equipment Packaging and Labeling Introduction Objectives A robust PQS is critical to assuring drug products are
Q10 Pharmaceutical Quality System Office of Communication Outreach and Development HFM 40 Center for Biologics Evaluation and Research Food and Drug Administration 1401 Rockville Pike Quality control in the pharmaceutical industry is often conflated with pharmaceutical quality assurance but they are different complementary approaches Where pharmaceutical quality control is largely reactive and product based focused on the testing of batches to ensure they meet quality objectives pharmaceutical quality assurance is a
More picture related to What Is Quality Control Department In Pharmaceutical Industry
Quality Pharmaceuticals Nippon Express
https://www.nipponexpress.com/img/industries/pharma/quality_img02.png

Top 10 Quality Control Department In 2023 Chuy n Trang Chia S Ki n
https://www.sourceofasia.com/wp-content/uploads/2022/02/Article-03.jpg

Introduction Of Quality Control Lab Quality Control Measures In
https://i.ytimg.com/vi/EP4cxwnUM9Q/maxresdefault.jpg
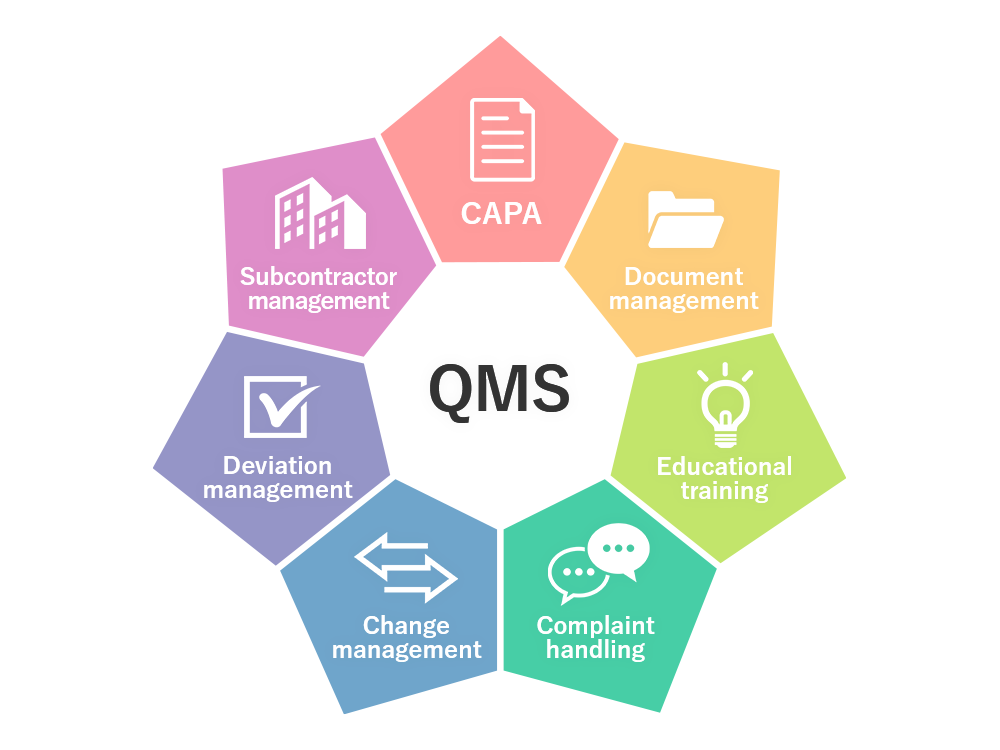
1 Drug and narcotic control standards 2 Drug industry standards 3 Pharmaceutical preparations standards 4 Biological products standards 5 Quality control 6 Guidelines I World Health Organization II Title Good manufacturing practices and inspection ISBN 92 4 154708 1 NLM classi cation QV 33 ISBN 978 92 4 154708 6 Quality control is an important part of quality management in a pharmaceutical setting On the other hand quality assurance is the process of making sure quality requirements have been fulfilled Quality assurance jobs aims to prevent mistakes and defects and manage quality through defining processes establishing standards and developing
Continuously performing the quality control management process re evaluating both the procedure and the product while keeping compliance in mind will result in a better final product happier customers and more profit At Amaris Consulting we offer a wide spectrum of services to pharmaceutical medical device and biotechnology companies Quality Control Is most Important part of Quality Team Quality Control Department is deal with Sampling Specification Analytical Procedure preparation appropriate execution Quality Control department is also documentation and release procedures which ensure that the necessary and relevant tests are carried out and that materials are not released for use nor products released for sale
Whose Responsibility Is Quality Management BrowserStack
https://browserstack.wpenginepowered.com/wp-content/uploads/2022/08/quality-management.png

QC Quality Control In Pharmaceutical Industry
https://1.bp.blogspot.com/-3HNdbgPUPgo/X6Z5qR1fb3I/AAAAAAAAJY0/-ejS5_cOomYcfDa0OHAffX626FiA1rKigCNcBGAsYHQ/s1920/QC%2B%2BQuality%2BControl%2Bin%2BPharmaceutical%2BIndustry.jpg
What Is Quality Control Department In Pharmaceutical Industry - The pharmaceutical quality control laboratory serves one of the most important functions in pharmaceutical production and control A significant portion of the CGMP regulations 21 CFR 211