What Are The Functions Of The Quality Control In Good Manufacturing Practice The main regulatory standard for ensuring pharmaceutical quality is the Current Good Manufacturing Practice CGMP regulations for human pharmaceuticals Consumers expect that each batch of
Quality managers can review the data to understand where manufacturing bottlenecks and waste exist in the process while retaining the quality needed Quality Control vs Quality Assurance Many people confuse quality control with quality assurance QA or use them interchangeably but they re two different concepts Quality control is the 9 Ensure Quality in Operations Manufacturing Logistics and Distribution Quality control should be embedded in every phase of the production and distribution process to ensure the final product meets the required standards 10 Perform Periodic Audits with Certified Auditors Conducting regular audits is the last of the ten principles of GMP
What Are The Functions Of The Quality Control In Good Manufacturing Practice
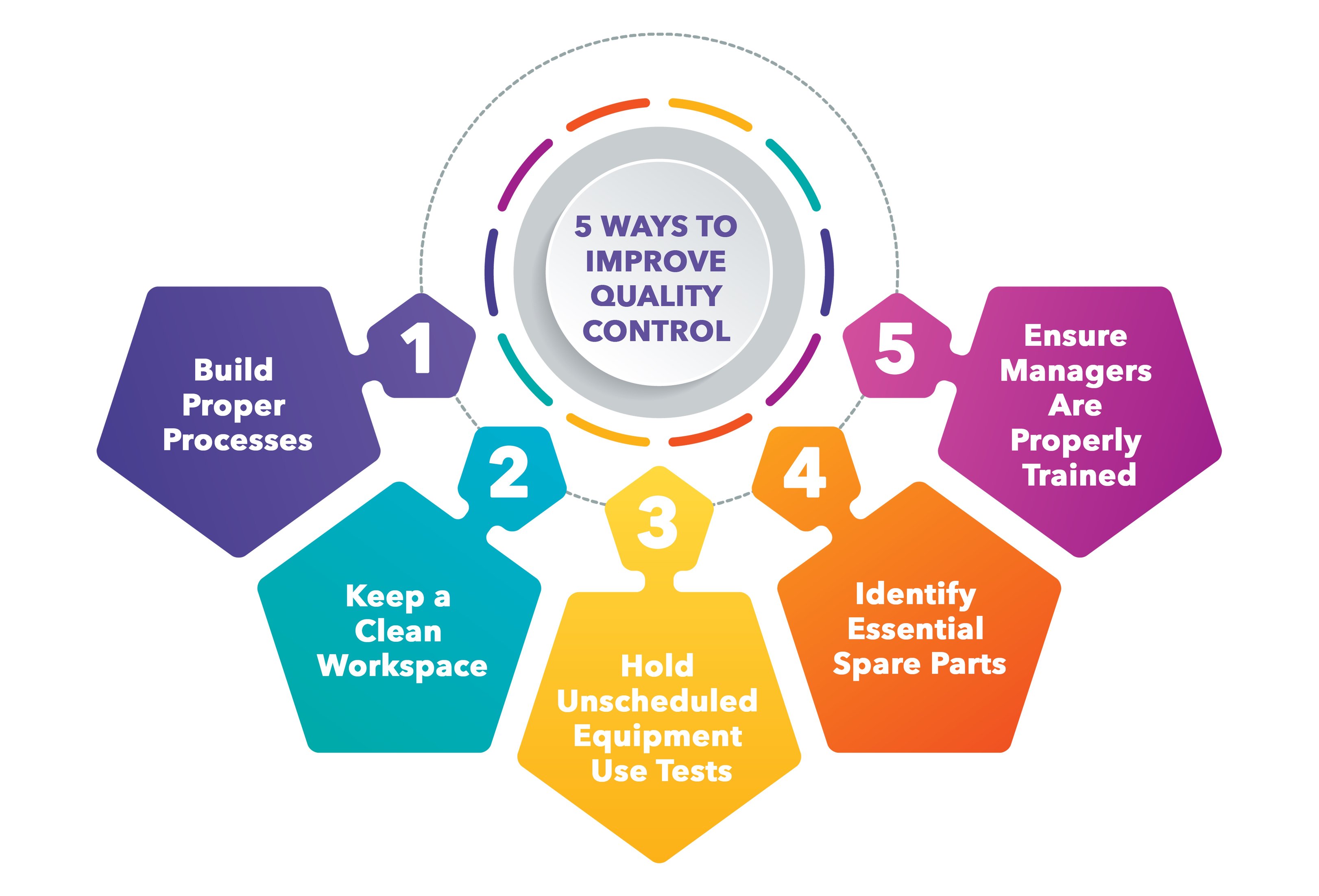
What Are The Functions Of The Quality Control In Good Manufacturing Practice
https://www.dozuki.com/hs-fs/hubfs/Imported_Blog_Media/Improve Quality Control in Manufacturing - infographic.jpeg?width=3243&name=Improve Quality Control in Manufacturing - infographic.jpeg

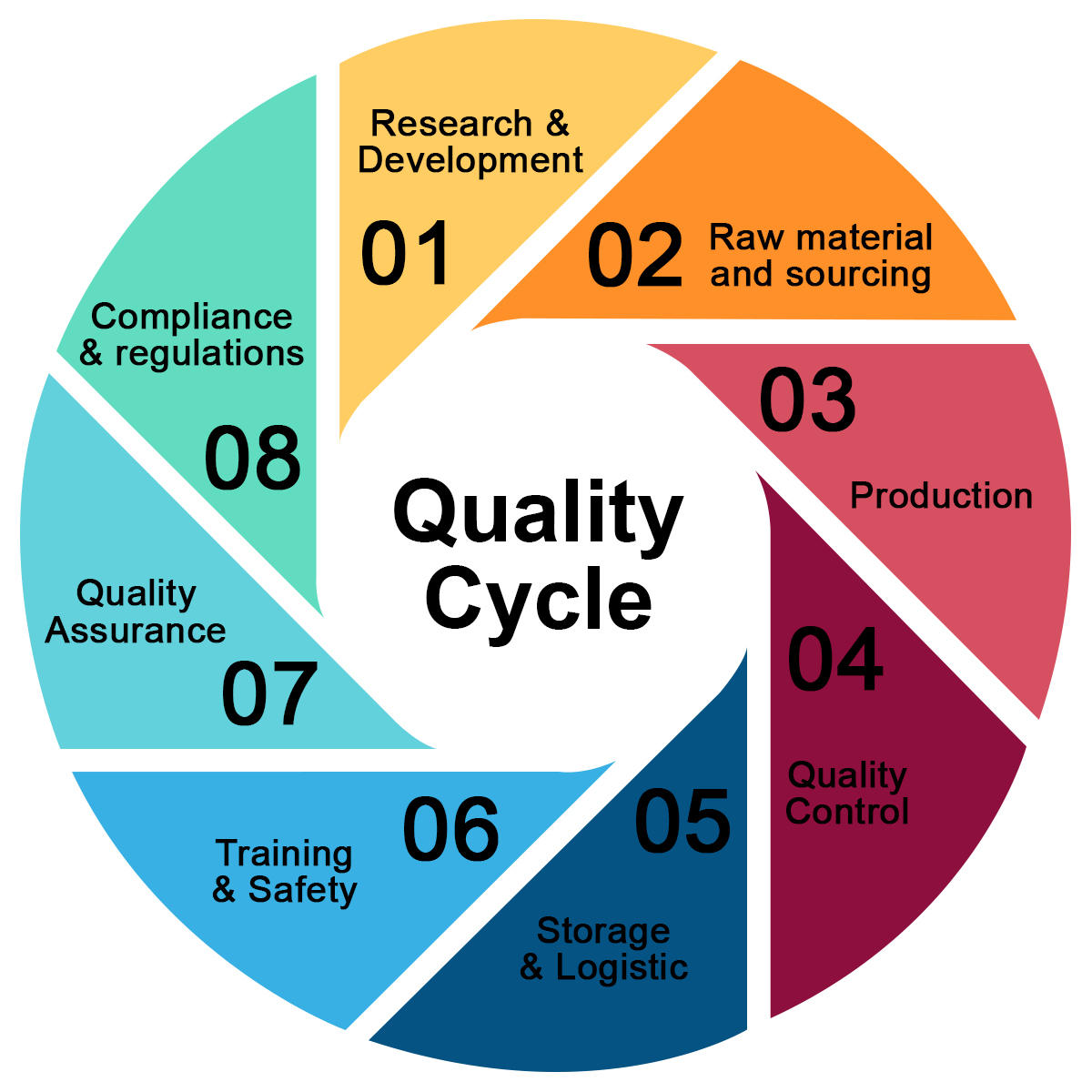
Quality Control Infographic 10 Steps Stock Vector Illustration Of
https://thumbs.dreamstime.com/z/quality-control-infographic-steps-quality-control-infographic-steps-concept-analysis-improvement-service-level-excellent-simple-158244736.jpg

Top 10 Quality Control Department In 2023 Chuy n Trang Chia S Ki n
https://www.sourceofasia.com/wp-content/uploads/2022/02/Article-03.jpg
The pharmaceutical or drug quality related regulations appear in several parts of Title 21 including sections in parts 1 99 200 299 300 499 600 799 and 800 1299 Good Manufacturing Practice for Products As a part of quality assurance good manufacturing practice is concerned with production and quality control It aims to mitigate the risks that are inherent in the production process Its basic requirements according to WHO s Good Manufacturing Practices for Pharmaceuticals can be found here Quality
The quality systems for FDA regulated products food drugs biologics and devices are known as current good manufacturing practices CGMP s CGMP requirements for devices in part 820 21 CFR Good Manufacturing Practices GMP also referred to as cGMP or current Good Manufacturing Practice is the aspect of quality assurance that ensures that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification GMP defines quality
More picture related to What Are The Functions Of The Quality Control In Good Manufacturing Practice

The Relationship Between Quality Assurance Quality Control And Good
https://i.pinimg.com/originals/ac/ad/7d/acad7ddca257e1370e494032849fae11.jpg
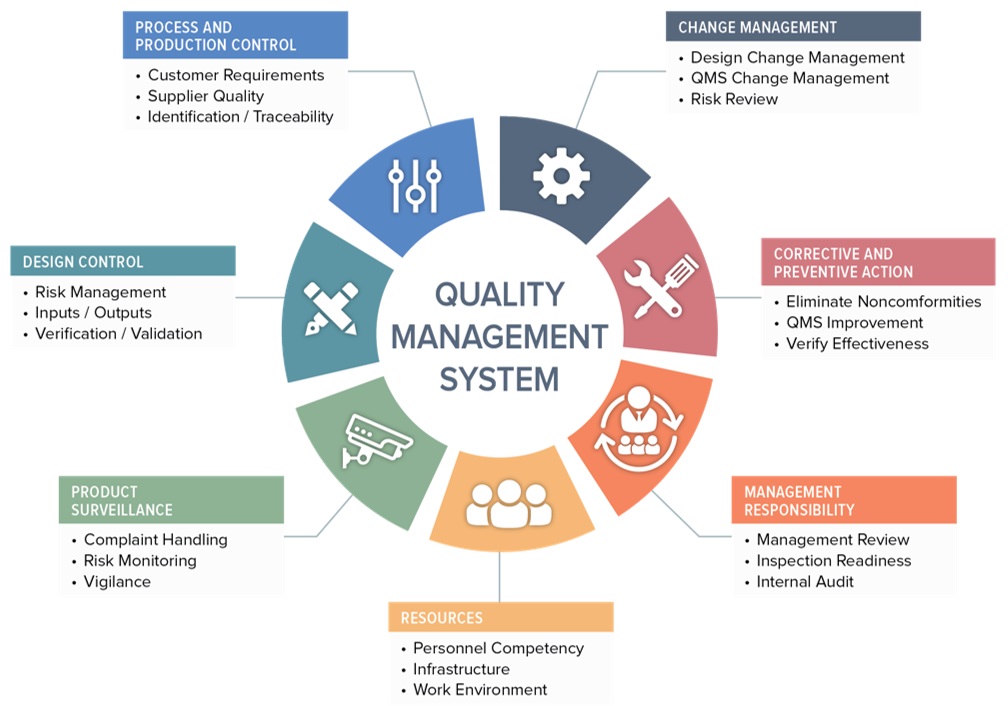
QMS Quality Management System Insightdeal in 2023
https://insightdeal.in/wp-content/uploads/2021/02/qms.jpg

THE Control OF Quality And The Functions Of The Quality Control
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/0dfadc09abc45b015f73e8b24c720541/thumb_1200_1553.png
Good Manufacturing Practices GMPs in the food industry are guidelines and principles implemented to ensure food safety and quality GMPs cover all aspects of food production from receiving raw materials to finished product shipment GMPs include guidelines on personnel hygiene plant sanitation equipment maintenance product labeling and Good Manufacturing Practice GMP is a system for ensuring that products are consistently produced and controlled according to quality standards It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product
Quality Control is that part of Good Manufacturing Practice which is concerned with sampling specifications and testing and with the organisation documentation and release procedures which Quality control plays a crucial role in ensuring that businesses deliver high quality products and services meeting customer expectations and regulatory requirements Companies can develop and implement effective QC systems that contribute to long term success by understanding its importance benefits and key strategies ISO 9001 2015 defines
Quality Control Tetrahedron
https://www.tetrahedron.in/wp-content/uploads/2020/04/Quality-Control.jpg

Quality Department Org Chart Org Charting
https://i.pinimg.com/originals/31/bc/51/31bc51ffe798ef1f1f8e6f01782a04b6.png
What Are The Functions Of The Quality Control In Good Manufacturing Practice - The specific regulations vary between jurisdictions but abiding by Good Manufacturing Practices GMP creates the baseline for safe and effective pharmaceutical manufacturing Drug manufacturers can make specific changes to abide by applicable regulatory requirements from there GMP best practices focus largely on quality assurance and quality