Fda Auditor Qualifications The purpose of this document is to define the Medical Device Single Audit Program MDSAP Team training requirements This procedure will assist to assure that MDSAP Team members have the
FDA Home Medical Devices Databases The information on this page is current as of Oct 17 2023 For the most up to date version of CFR Title 21 go to the Electronic Code of Federal Regulations About This Course Gain valuable knowledge on the practical application of the requirements specific to the U S FDA requirements of 21 CFR Part 820 Learn audit program management including preparing internal audit plans and how to address gaps during the audit
Fda Auditor Qualifications
:max_bytes(150000):strip_icc()/GettyImages-1180593804-717c7ae8c41449d88fdf301778505628.jpg)
Fda Auditor Qualifications
https://www.investopedia.com/thmb/wbodJ6H3ImdlD6wjGRGWsgKR4DA=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/GettyImages-1180593804-717c7ae8c41449d88fdf301778505628.jpg

Best Guide On How To Become Health Information Technician
https://www.goclassroom.org/wp-content/uploads/2023/01/pexels-artem-podrez-5726697-scaled.jpg

Aprilista Rines Prananda Senior Auditor HLB Hadori Sugiarto Adi
https://media-exp1.licdn.com/dms/image/C4E16AQE3ISLRSKb0bQ/profile-displaybackgroundimage-shrink_200_800/0/1624642631010?e=2147483647&v=beta&t=IoPOJ-ACAmTZ0wRMid47nzPOlxos4a-v-pJMNg3NUOQ
Support all claims and actions with facts Include documents that address actions training logs process revisions meeting sign in sheets etc There are several elements within the FDA audit process for which the investigator and research site will need to prepare and act upon before the audit during the audit and after the audit As such an FDA auditor is someone who must have a balance of specific education and relevant work experience He is not only educated regarding aspects of chemistry biology and medicine but
Requirements for Recognized Accreditation Bodies Sec 1 1125 What are the internal audit requirements for a recognized accreditation body As part of the internal audit a recognized accreditation body is required to conduct pursuant to 1 1113 a the recognized accreditation body must audit its compliance with the requirements of 1 1113 d Robert Ruff Rob has over 30 years experience in the medical device and public health sectors including completing a long distinguished career with the U S FDA His extensive regulatory experience includes co authoring the MDSAP Audit Model and the Quality System Inspection Technique QSIT He also developed FDA s computer based training for QSIT the Quality System Regulation and
More picture related to Fda Auditor Qualifications

Justin L Quality Director Master Of SSBB XXX Household Electric
https://media.licdn.com/dms/image/C4D03AQGxndm4A5yYzw/profile-displayphoto-shrink_800_800/0/1668044572491?e=2147483647&v=beta&t=xEcMpNjmaNye9JNAGaARrrWY2t447wQLKr7XVQKPGDU

Zolt n Horv th Production And Technology Manager Polifoam Kft
https://media-exp1.licdn.com/dms/image/D4D03AQEDhze1dyfpmg/profile-displayphoto-shrink_800_800/0/1667628139289?e=2147483647&v=beta&t=Mkj1l2L0S2qrQkUTthGILOtfCfdwNYzm0iMrazASZa8
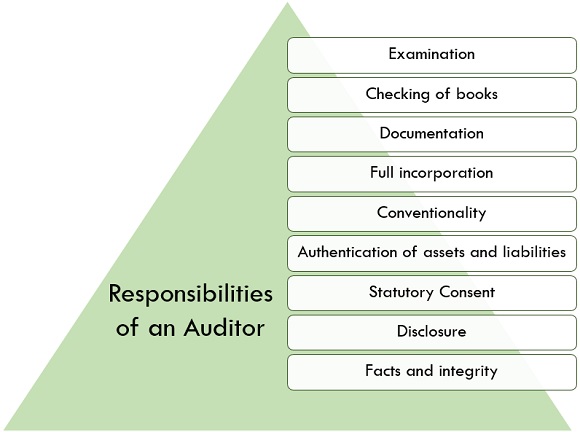
Who Is An Auditor Qualifications Qualities Responsibilities And
https://theinvestorsbook.com/wp-content/uploads/2020/04/responsibilities-of-an-auditor-1.jpg
Ensure auditor qualifications and training Developing an audit schedule Risk Based Preparation for the audit developing audit conduct tools The Course Director did an outstanding job explaining FDA style audits as was what to look for when performing an audit internal or external This is the best quality auditing course I have 3 The audit must be sufficiently rigorous to allow the accredited third party certification body to determine whether the eligible entity is in compliance with the applicable food safety requirements of the FD C Act and FDA regulations and for consultative audits also includes conformance with applicable industry standards and practices at
The FDA agents conducting your company s inspection or audit will dutifully fulfill their role and they will not leave any relevant stone unturned 3 Prepare Prepare Prepare Next it is An FDA audit requires significant preparation since inspectors will be going through your quality systems thoroughly to make sure you re following federal GMP regulations Problems with an audit can lead to costly corrective actions plant shutdowns or even more severe consequences

Our Lead Auditor Muhammad Ali Managing Director Registered Lead
https://wwise.co.za/wp-content/uploads/2020/04/WWISE2020Jan021.jpg

More Companies Seek Legal Views As Auditor Qualifications Rise Flipboard
https://img.etimg.com/thumb/msid-72972966,width-1070,height-580,imgsize-430756,overlay-economictimes/photo.jpg
Fda Auditor Qualifications - Reporting requirements for internal audits Part 2 Regulatory Inspections How to prepare for a visit from an FDA Auditor Instructor Joy McElroy Areas Covered in the Webinar The purpose and scope of FDA audits FDA forms 482 and 483 What exactly a QSIT audit entails and how to pass it